
Therefore, calculating the molar mass of the hydrogen atoms in H 2 O is. Thus, the molar mass equation must account for a compound’s number of atoms of a certain element. If the molar mass of hydrogen is 1.008 g/mol, what is the mass (in grams) of 2 hydrogen atoms A. In a water molecule, two hydrogen atoms are present alongside one oxygen atom. For a compound like water (H2O), 1 mole of hydrogen (H) is 1.008 g/mol and 1. This molar mass calculator can only handle two bracket levels at a time. The atomic mass of hydrogen (H) is 1.00784, and the atomic mass of oxygen (O) is 15.9994. The molar mass is the mass in grams of 1 mole of a particular molecule. Tricalcium phosphate would be entered as Ca3(PO4)2.
For example, calcium carbonate would be entered as CaCO3, not caco3.

The chemical formula should be entered using standard format. This calculator is a convenient tool for calculating the molar mass of chemical compounds in lieu of using a periodic table. These include consumption of pH adjustment chemicals for RO feedwater, solubilities of scale forming compounds in reverse osmosis systems, and cation rejection calculations using charge balance (meq/l) in nanofiltration systems. Many other calculations require conversion into moles. If 10.0 grams of nitrogen gas combines with 10.0 grams of hydrogen gas, find the following: the molar mass of reactants and products, the limiting reactant, the excess reactant, the amount of ammonia produced, the amount of excess chemical not used in the reaction. See also our theoretical yield calculator for chemical reactions (probably your next stop to finish the problem set). Question: What is the molar mass of hydrogen gas 1.20 x 1023 g/mol 1.01 g/mol 2.02 g/mol 6.02 x 1023 g/mol. The Haber Process involves nitrogen gas combining with hydrogen gas to produce ammonia.
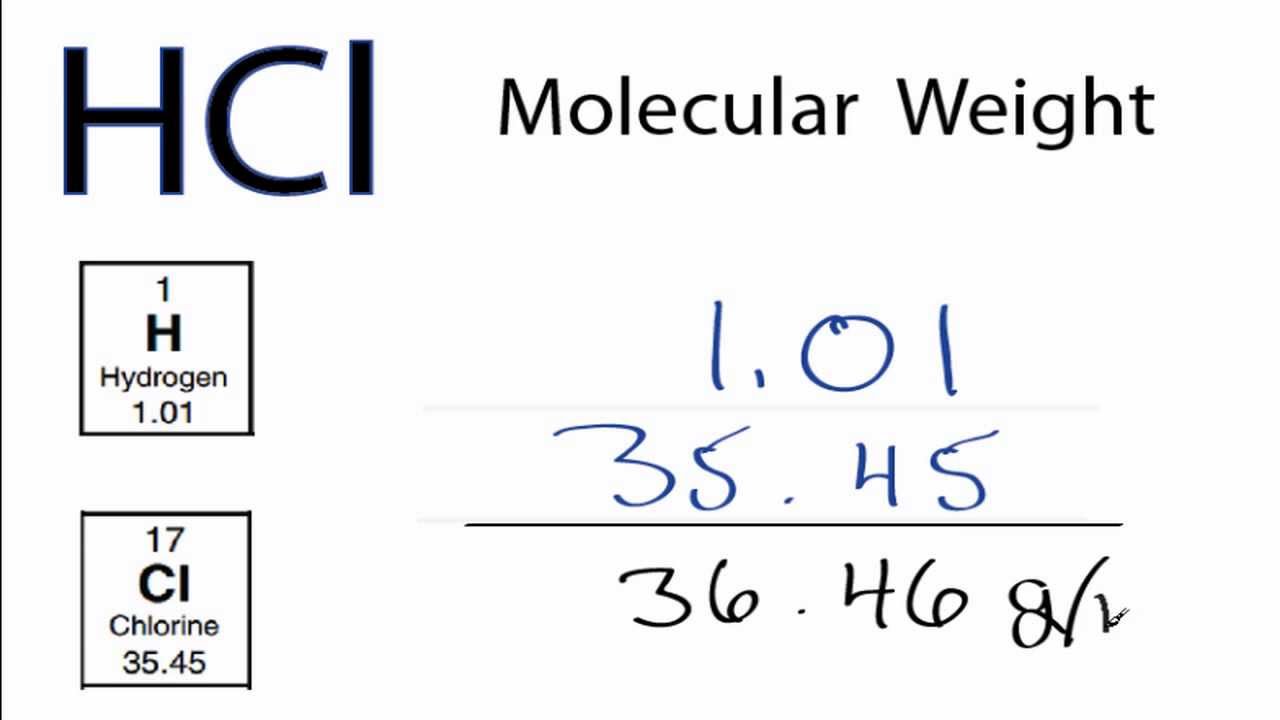
Use this visual tool for calculating molar mass for any chemical formula. The molar mass of Na2HPO4 (Sodium hydrogen phosphate) is: 141.955 grams/mol. Chemical formula (Hill notation) Molar mass (g/mol) Modify Clear mnM. Knowing the desired concentration of ClO2, the system integrator can calculate the consumption of each of the reactants using the stoichiometric relationship:ĢNaClO2 + NaOCl + 2HCl ↔ 2ClO2 + H2O + 3NaCl Select elements and see the molar mass of the compound. For example, certain types of chlorine dioxide (ClO2) generators would use sodium hypochlorite (NaOCl), sodium chlorite (NaClO2) and hydrochloric acid (HCl). When calculating consumption of certain RO chemicals for reverse osmosis pretreatment or post-treatment, it is often necessary to convert into moles.


 0 kommentar(er)
0 kommentar(er)
